|





|
5.
Department of Materials Chemistry (Rudjer Bošković Institute, Zagreb)
Svetozar Musić
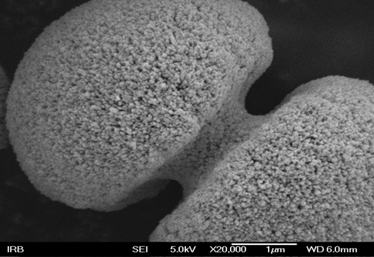
Almost four decades ago dr.sc. Svetozar Musić introduced at the Rudjer
Bošković Institute, Zagreb, the research in chemistry of metal oxides.
This research started with the preparation of
wo
interconnected cupolas of a-Fe2O3 precipitated
from dense a-FeOOH suspension with added ammonium amidosulfonate (M.
Žic, M. Ristić, S. Musić, J. Mol. Struct. 924-926 (2009)
235)metal
oxide carriers labelled with cyclotron radioisotopes for applications in
nuclear medicine and it was followed with the investigation of the
hydrolytic processes and precipitation of iron oxides. With time a
research group in chemistry of metal oxides was formed which started a
systematic investigation of other metal oxide systems such as, ZrO2,
TiO2, ZnO, Ga2O3, Nb2O5,
WO3 etc. The aim of these investigations was to ascertain the
relationship between the synthesis route and specific properties of
metal oxide particles. The kinetics and mechanisms of the precipitation
of metal oxides were investigated. The formation of solid solutions in
different metal oxide systems was also determined. The formation and
properties of ferrites with spinel-, perovskite- or garnet-type
structure were investigated. In all these investigations a particular
attention was focused to the formation of nanostructured metal oxides.
The application of X-ray powder diffraction in combination with other
techniques (Mossbauer, FT-IR, Raman, electron microscopy) made possible
to study the structure, phase composition, phase diagrams, chemical
bonds, as well as the size and morphology of the particles of metal
oxides (e.g. J.Mater.Sci. 24(1989)2722; Mater.Letters
23(1995)139; J.Mater.Sci. 31(1996)4067; J.Alloys Comp.
241(1996)10; Thermochim.Acta 303(1997)31;
Croat.Chem.Acta
71(1998)789; J.Non Cryst.Solids 303(2002)270; J.Alloys
Comp. 398(2005)188; 429(2007)242, J. Mol. Struct.
924-926(2009)243).
Picture: Two interconnected cupolas of a-Fe2O3
precipitated from dense a-FeOOH suspension with added ammonium
amidosulfonate (M. Žic, M. Ristić, S. Musić, J. Mol. Struct. 924-926
(2009)
235) |