|





|
7.1 Laboratory of Solid State
Chemistry, Rudjer Bošković Institute, Zagreb
Želimir
Blažina
Starting
with the late fifties of the past century, crystallography has a long
tradition in the Laboratory for Solid State Chemistry (former Laboratory
for High Temperature Materials) of the Rudjer Bošković Institute in
Zagreb. Numerous techniques used for preparation and identification of
crystalline materials have been developed and applied since then. Single
crystal growth of Rochelle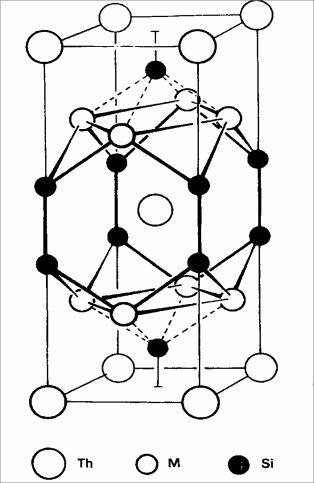 salt, influence of impurities on its crystal morphology, incorporation
of impurities into the crystal and their effect on (di)electric
properties were studied (M. Topić). About that time float zone crystal
growth was used for preparation of high purity single crystals of
germanium and silicon. Some year later uranium and thorium ternary
silicides and germanides containing transition metals were prepared, and
their crystal structures were studied by X-ray powder diffraction.
Previously unknown crystal structure type of the composition ThM2Si2
was determined (Z. Ban and M. Sikirica). X-ray powder diffraction and
neutron diffraction methods were used to study the irreversibly phase
transition of cubic thorium carbohydride (carbodeuteride) ThCH2(D)
into its hexagonal modification which was observed at 380oC
(M. Makovec). The discovery of fcc allotropic modification of uranium
and its stabilisation by small amounts of non-metals (S, N, O, Se, Te)
was also studied using neutron and X-ray powder diffraction methods. (M.
Tudja). Chemical vapour transport technique was used for preparation of
ternary uranium compounds of the composition UNTe, UNAs and UAsSb.
Their crystal structure was determined by X-ray powder diffraction; it
was found that they crystallised in the tetragonal structure of the
PbFCl type (Z. Despotović, R. Trojko). The technique of isothermal
transport reactions (with or without initial contact) between metals in
molten alkali chloride mixtures was developed, and applied to the
systems containing combinations of Cu, Ni, Ag, Pt, Cr and Fe metals (M.
Paljević). The ongoing intensive research, preparation, stability
studies and crystal structure determination of multicomponent
intermetallic compounds started with the early seventies of the past
century. A systematic study of phase equilibrium was carried out for
selected rare earth - iridium systems. Substitution of one or both
components in binary intermetallic compounds of the general composition
AB2 and AB5 with other metals or metalloids, has
been used as a standard method for tailoring materials suitable for
hydrogen storage purposes. Thermodynamic properties of the corresponding
intermetallic compound – hydrogen systems were determined from the
pressure composition isotherms (PCI) at various temperatures obtained by
tensimetric methods (Ž. Blažina, A. Drašner, B. Šorgić). So far the
crystal structures of about fifty intermetallic compounds have been
quoted in Pearson’s Handbook of Lattice Spacings and Structures of
Metals, in the Powder Diffraction File Search Manual of Inorganic
Compounds and in Metal Hydride Reference List of Sandia National
Laboratories. The most recent intensive investigations are theoretical
studies of intermetallic compounds based on electronic structure
calculations. The standard calculation methods within the density
functional theory (DFT) are used. The aim is to obtain theoretical
parameters which will elucidate the fundamental knowledge of
thermodynamic, electrical, chemical and magnetic structure and
properties of this class of materials (G.Miletić).
salt, influence of impurities on its crystal morphology, incorporation
of impurities into the crystal and their effect on (di)electric
properties were studied (M. Topić). About that time float zone crystal
growth was used for preparation of high purity single crystals of
germanium and silicon. Some year later uranium and thorium ternary
silicides and germanides containing transition metals were prepared, and
their crystal structures were studied by X-ray powder diffraction.
Previously unknown crystal structure type of the composition ThM2Si2
was determined (Z. Ban and M. Sikirica). X-ray powder diffraction and
neutron diffraction methods were used to study the irreversibly phase
transition of cubic thorium carbohydride (carbodeuteride) ThCH2(D)
into its hexagonal modification which was observed at 380oC
(M. Makovec). The discovery of fcc allotropic modification of uranium
and its stabilisation by small amounts of non-metals (S, N, O, Se, Te)
was also studied using neutron and X-ray powder diffraction methods. (M.
Tudja). Chemical vapour transport technique was used for preparation of
ternary uranium compounds of the composition UNTe, UNAs and UAsSb.
Their crystal structure was determined by X-ray powder diffraction; it
was found that they crystallised in the tetragonal structure of the
PbFCl type (Z. Despotović, R. Trojko). The technique of isothermal
transport reactions (with or without initial contact) between metals in
molten alkali chloride mixtures was developed, and applied to the
systems containing combinations of Cu, Ni, Ag, Pt, Cr and Fe metals (M.
Paljević). The ongoing intensive research, preparation, stability
studies and crystal structure determination of multicomponent
intermetallic compounds started with the early seventies of the past
century. A systematic study of phase equilibrium was carried out for
selected rare earth - iridium systems. Substitution of one or both
components in binary intermetallic compounds of the general composition
AB2 and AB5 with other metals or metalloids, has
been used as a standard method for tailoring materials suitable for
hydrogen storage purposes. Thermodynamic properties of the corresponding
intermetallic compound – hydrogen systems were determined from the
pressure composition isotherms (PCI) at various temperatures obtained by
tensimetric methods (Ž. Blažina, A. Drašner, B. Šorgić). So far the
crystal structures of about fifty intermetallic compounds have been
quoted in Pearson’s Handbook of Lattice Spacings and Structures of
Metals, in the Powder Diffraction File Search Manual of Inorganic
Compounds and in Metal Hydride Reference List of Sandia National
Laboratories. The most recent intensive investigations are theoretical
studies of intermetallic compounds based on electronic structure
calculations. The standard calculation methods within the density
functional theory (DFT) are used. The aim is to obtain theoretical
parameters which will elucidate the fundamental knowledge of
thermodynamic, electrical, chemical and magnetic structure and
properties of this class of materials (G.Miletić).
*
on the picture:
ThM2Ge2 structure, Acta
Cryst 18 (1965) 584
7.2 The Glass
Laboratory, Rudjer Bošković Institute, Zagreb
Andrea Moguš-Milanković
The Glass Laboratory at
Rudjer Bošković Institute (Andrea Moguš-Milanković, Ana Šantić, Luka
Pavić) employs crystallography in conjunction with electrical
characterization to study relationship between the composition,
structural and electrical properties of various materials. Principal
techniques in electrical characterization include the Impedance
Spectroscopy (IS) and Thermally Stimulated Polarisation Current (TSDC/TSPC)
measurements combined with Raman spectroscopy and XRD techniques.
Research programs cover transition metal
oxide glasses, bioactive materials and ionic liquids composites.
Special interest is focused on the mechanism
of crystallization of amorphous systems (phosphate, silicate, bioactive
glasses), determination of crystalline phases, particle sizes and volume
fractions and their influence on the electrical properties. Part of the
research considers a surface activity of the electrically polarised
bioactive materials.
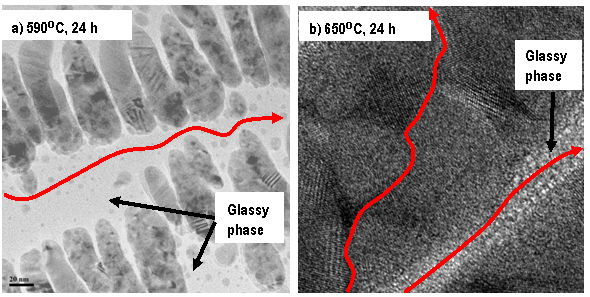
Easy conductive pathways in partially crystallized 40Fe2O3-60P2O5
(mol%) glass through a) glass matrix and b) grain boundaries |